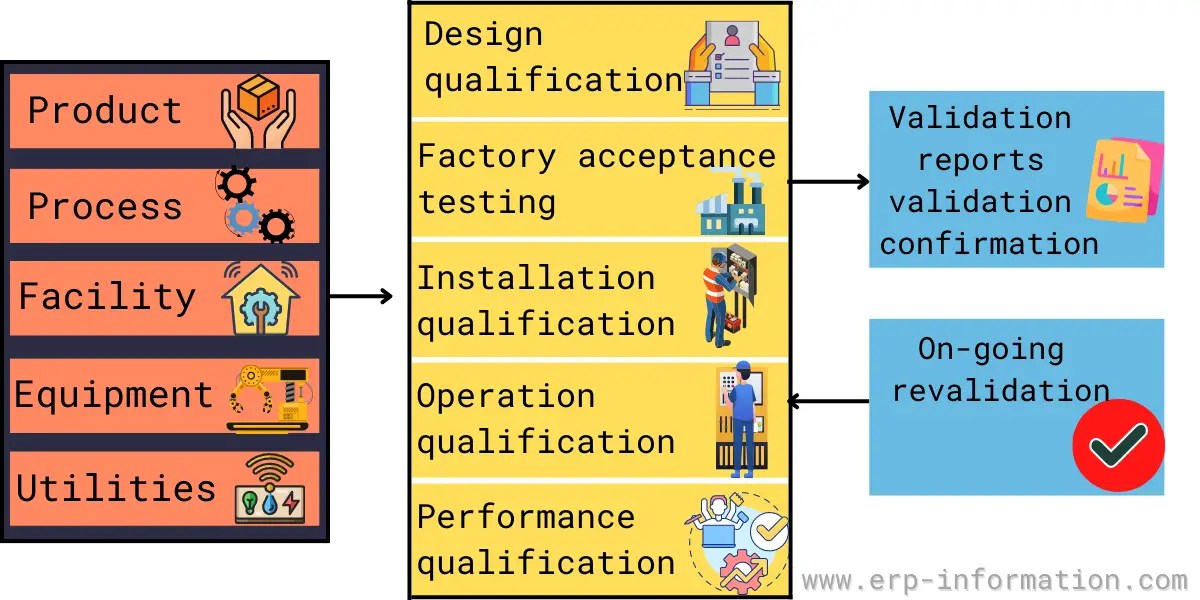
Master Validation Plan Template - Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to be produced in order to ensure full consideration is given to product quality aspects. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. The validation master plan (vmp) is a summary of the planned validation activities. It lists those activities and essential. A procurement approach introduction to master validation plans. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web three (3) options to create a validation master plan. Web master validation plan template for medical devices:
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to be produced in order to ensure full consideration is given to product quality aspects. Web master validation plan template for medical devices: Saxton this article describes the elemental.
Validation Master Plan Template Verification And Validation Pharmaceutical
Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web three (3) options to create a validation master plan. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each.
How to create a Validation Master Plan in 5 steps. Templates & more
The validation master plan (vmp) is a summary of the planned validation activities. A procurement approach introduction to master validation plans. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to be produced in order to ensure full.
Overview of the Validation Master Plan PresentationEZE
Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. A procurement approach introduction to master validation plans. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web three (3) options to create a validation master plan. Web a validation master.
Validation Master Plan (VMP) Downloadable Interactive Template.
Web master validation plan template for medical devices: It lists those activities and essential. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. The validation master plan (vmp) is a summary of the planned validation activities. Web three (3) options to create a validation master plan.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
A procurement approach introduction to master validation plans. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. Web three (3) options to create a validation master plan. Web master validation plan template for medical devices: Web a master validation plan (mvp) is a plan for your equipment.
What is Validation Master Plan? (Template, Examples)
Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. The validation master plan (vmp) is a summary of the planned validation activities. Web master validation plan template for medical devices: Web.
Master Validation Plan Template
The validation master plan (vmp) is a summary of the planned validation activities. It lists those activities and essential. Web three (3) options to create a validation master plan. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web a master validation plan.
Nine steps for creating a Master Validation Plan
It lists those activities and essential. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to.
Validation Master Plan Template Validation Center
The validation master plan (vmp) is a summary of the planned validation activities. Web master validation plan template for medical devices: Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web three (3) options to create a validation master plan. A procurement approach.
Web a master validation plan (mvp) is a plan for your equipment and process validation activities. It lists those activities and essential. Web master validation plan template for medical devices: A procurement approach introduction to master validation plans. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. The validation master plan (vmp) is a summary of the planned validation activities. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web three (3) options to create a validation master plan. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to be produced in order to ensure full consideration is given to product quality aspects.
The Validation Master Plan (Vmp) Is A Summary Of The Planned Validation Activities.
Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. It lists those activities and essential. Web master validation plan template for medical devices: Web a master validation plan (mvp) is a plan for your equipment and process validation activities.
Web Master Validation Plan Is A Strategic Document Which Identifies The Elements To Be Validated, The Approach To Be Taken For Validation Of Each Element, The Organizational Responsibilities And The Documentation To Be Produced In Order To Ensure Full Consideration Is Given To Product Quality Aspects.
Web three (3) options to create a validation master plan. A procurement approach introduction to master validation plans. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the.